Background
Tisagenlecleucel and brexucabtagene autoleucel have demonstrated remarkable efficacy and safety results in adult patients with relapsed/ refractory acute B lymphoblastic leukemia (R/R B-ALL). Long term follow-up of the ZUMA-3 trial recently reported a median duration of response (DOR) of 14.6 months and an overall response rate of 71% (Hadjivassileva, EBMT 2023).
Methods and patients
We report results of a phase 1b/2 single center clinical trial (NCT02772198) involving point-of-care (POC) anti-CD19/CD28 costimulatory domain chimeric antigen receptor (CAR) T-cell therapy in adults with R/R B-ALL. Inclusion criteria were age ≥18 years, failure of at least 2 prior therapies, and a preserved organ function. Patients underwent a single leukapheresis procedure. Fresh CAR T products were delivered for immediate infusion. Lymphodepletion included fludarabine and cyclophosphamide. Cell dose was 1x10 6 CAR T cells/kg. Primary endpoints were 1-month disease response and safety. Secondary endpoints were the minimal residual disease (MRD) negativity rate, overall survival, progression-free survival (PFS), and production feasibility. Last follow-up was July 2023.
Results
Between 03/2017-05/2023, 28 patients enrolled. CAR T-cells were successfully produced in 27 patients (96%) who were included in the analysis. The median age was 33 years (range 19-77), and 7 (25%) patients had Karnofsky Performance Status <90%. Six patients (22%) had positive Philadelphia chromosome, and 14 patients (52%) had an extramedullary disease at leukapheresis. Nineteen patients (70%) had ≥3 prior lines of therapy, 18 patients (67%) underwent prior allogeneic hematopoietic stem-cell transplantation (allo-HCT), and 20 patients (74%) had prior exposure to inotuzumab-ozogamicin or blinatumomab. At leukapheresis, 16 patients (60%) had an active disease, while 11 patients (40%) were in complete morphological response (CR; MRD positive, n=7 [26%], MRD negative or not evaluated, n=4 [15%]).
The median time between leukapheresis and cell infusion was 11 days (IQR 10-11). Only 3 patients (11%) received bridging chemotherapy. Grade 3-4 cytokine release syndrome and immune effector cell-associated neurotoxicity syndromes were observed in 30% of the patients, each. Six (22%) and 16 (59%) patients were treated with tocilizumab or corticosteroids, respectively. Severe neutropenia (<0.5k/μl) and thrombocytopenia (≤50 K/μl) occurred in 81% and 70% of the patients, respectively, and anemia requiring blood transfusion occurred in 71%. Three patients (11%) had grade 3 cardiovascular events, and 1 patient (4%) had grade 3 pleural effusion. Bloodstream bacterial infection occurred in 3 (11%) patients. Cellular therapy-related mortality was observed in 2 (7%) patients (both due to septic shock).
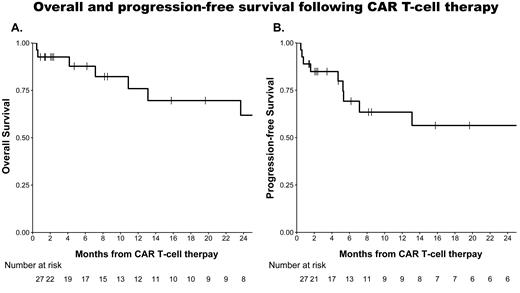
The 1-month overall response rate was 88% (81% CR; MRD negative CR, 63%). MRD was evaluated by RT-PCR in most of the patients. The median follow-up was 19.7 months (IQR 4.7-43.2). Two-year overall survival and PFS were 62% (95% CI: 42-91), and 56% (95% CI: 38-84), respectively. The median DOR was not reached (95% CI: 6.1 months-not reached). PFS was not affected by prior exposure to inotuzumab-ozogamicin or blinatumomab (hazard ratio (HR) 0.7, 95% CI: 0.17-2.68, p=0.6). All eligible patients who achieved a response were offered consolidation with allo-HCT. Eight patients (30%) underwent allo-HCT (2 nd allo-HCT in 3 of them). The DOR was not affected by consolidation of CR with allo-HCT (HR 0.9, 95% CI: 0.2-4.1, p>0.9). Nevertheless, 2 patients (7%) died due to allo-HCT related complications while in CR.
Conclusion
Treatment with POC CAR T-cells, produced in an academic center, achieved high response and survival rates in adults with R/R B-ALL. These results are also comparable to the FDA approved CAR T-cells. The use of POC CAR T abrogates the need for cryopreservation and shipment of the cells, thus allowing a short vein-to-vein time without the need of bridging therapy in most patients.
Disclosures
Jacoby:Novartis: Honoraria, Speakers Bureau; Medison: Speakers Bureau. Avigdor:BMS: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel/Accommodations/Expenses; Takeda: Membership on an entity's Board of Directors or advisory committees; MSD: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal